The First Principal Energy Level Is Called the
Commutator Uncertainty Principle 25 Chapter 3. The element is helium.
How Many Sublevels Are In The Second Energy Level At Level
Is means that there are two electrons in the s orbital of the first energy level.
. The block depends on which sublevel is in the process of being filled. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. There are shaped like a 3D figure of eight.
The orbits have quantized sizes and energies. Up to 24 cash back For 1st energy level n 1Maximum number of electrons in 1st energy level 2n22 x 1 2 2 For 2nd energy level n 2Maximum number of electrons in the 2nd energy level 2n22 x 22 2 x 4 8 For 3rd energy level n 3Maximum number of electrons in the 3rd energy level 2n2 2x3 2 2 x 9 18. The third energy level has s p and d sublevels.
Now putting the values of n and Z we get the Bohr radius as. Radioactive Decay Part I PDF - 10MB 7 Tunneling scattering 3132 Lecture 7 slides PDF - 11MB 8 Alpha decay 33 Chapter 4. The s - Sublevel can hold a maximum of.
While according to Quantum. E n R h 1 n 2. This contrasts with classical particles which can have any amount of energy.
The -forth energy level has s p d and sublevels. State One Fact of Bohrs Model. Select oneaPrinciple one state bGround state cAtomic state dHunds state Feedback The correct answer isGround state 2.
The 3s orbital differs from the 2s orbital in that it is. The energy of an electron when it is far away from the influence of the nucleus is taken as zero. An S-Orbital in the first energy level is a 1s orbital.
Up to 24 cash back Every energy level contains one S-Orbital. An S-Orbital in the second energy level is a 2s orbital etc. Question text The first principle energy level is called the.
The group is the vertical column. Each Principle Energy Level is comprised. Principal Energy Level of Sublevels.
A number indicates the energy level The number is called the principal quantum number. 5s 5p 5d 5f 5g. The d - Sublevel can hold a maximum of.
Shown here is the first Balmer transition in which an electron jumps from orbit n 3 to orbit n 2 producing a photon of red light with an energy of 189 eV and a wavelength of 656 nanometres. The period number corresponds to the main energy level that is filling with electrons which make up the valence shell. Energy Levels PDF 9 Bound systems and energy levels 41 10 Angular momentum 42 Lecture 10 slides PDF 11 Angular momentum.
As it is in second orbit so n 2. Where R H is called Rydberg constant whose value is 21810 18 J. You may have noticed in writing electron configurations that the s sublevel of a principal energy level n is always occupied before d electrons are added to the principal energy level numbered n - 1.
We know that formula for Bohr radius r r0n2Z. An electron is excited from the n1 ground state to the n3 state in a hydrogen atom. The highest occupied principal energy level is the fifth so this element is in Period 5.
The first energy level has an s sublevel. What is the first principle energy level called Get the answers you need now. A model of an atom shows eight electrons in rings that represent different energy levels.
Sum rules 12 Hydrogen atom 43 13. The first principle energy level is called the. The next shell has a value of n2 etc.
Principle one state b. An element with 8 electrons in its outermost main energy level is called a. Up to 24 cash back 1.
The first principal energy level of the hydrogen atom contains only an. This level is denoted by the principal quantum number n. The second energy level has s and p sublevels.
4s 4p 4d 4f. Sylviaruthwilson1971 sylviaruthwilson1971 1 week ago Chemistry College answered What is the first principle energy level called 2 See answers Advertisement Advertisement freshman14 freshman14 Answer is s orbital. The valence electrons are those in the outermost principal energy level.
Every energy level except the first level contains three P-Orbitals. A superscript indicates the number of electrons in the orbital. GENERAL CHEMISTRY 1 LAB EXAM 5 The first principle energy level is called the.
Their valence electrons are in the first energy level ncolorred1 as is denoted by colorred1s1 and color red1s2. The first energy level is also called level K. Ex 4s fills before 3d because 4s has less energy than 3d.
All p orbitals are shaped like. A letter indicates the type of orbital. Each shell has a different energy level increasing the further it is from the nucleus.
For name and type of element use a periodic table. The maximum number of electrons possible in the first four energy levels are. Of different Sublevels which are.
Electrons in the outermost energy level are also called Valence electrons. They exist in groups of three. The correct sequence in ascending energies of atomic sublevels is.
We review their content and use your feedback to. How many electrons are in each energy level1 point 1two in the first energy level six in the second energy level 2eight in the first. S p d f.
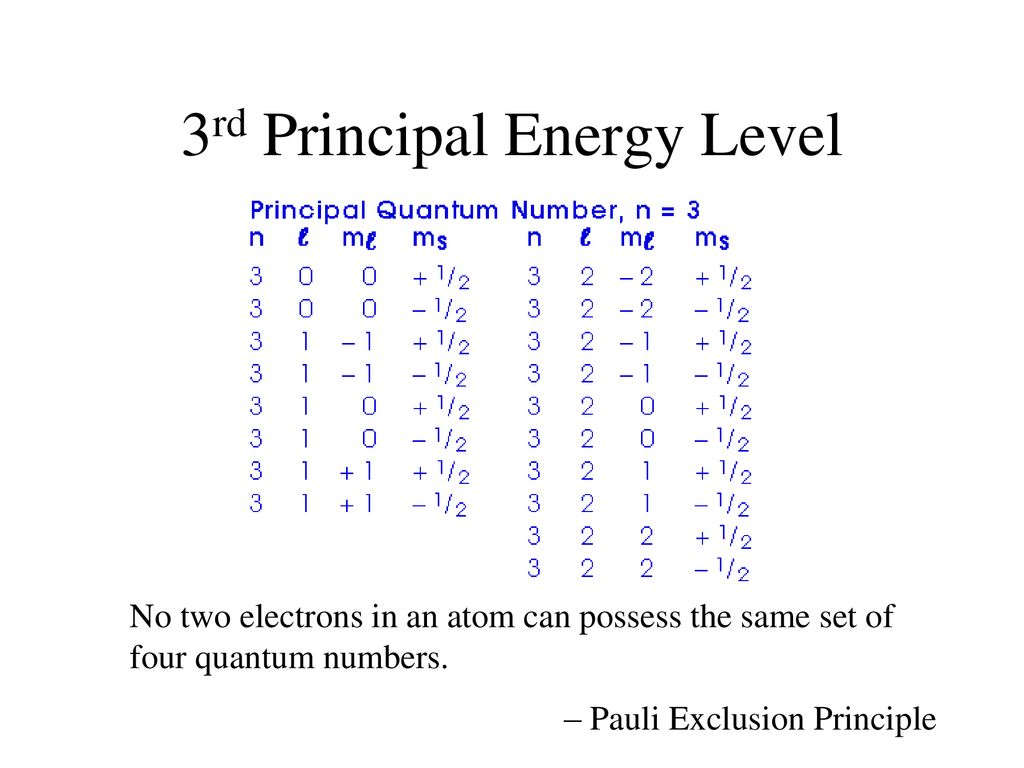
0 M LJLJ n jsubJevels inside l s 2- s p 3 S p d 4- s p d Notice that the number o sublevels in an energy level o the energy level. Yes the 5th energy level holds 5 sublevels and that last one would be 5g. The first element in a period of the.
They make up the first period row of the periodic table. The first four energy levels are shown here. The term is commonly used for the energy levels of the electrons in atoms ions or molecules which are bound by the electric field of the nucleus but.
In chemistry the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atoms nucleus. Each energy level is given a number called the principal quantum number n. Experts are tested by Chegg as specialists in their subject area.
Principal quantum number of an electron existing in such a stationary state is taken as n. The closest shell has a value of n1. The p - Sublevel can hold a maximum of.
The second level is called level L third energy level as M and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy. The Principal Energy Level the only holds that of sublevels.
S p d f. Who are the experts. Niels Bohr model of atoms says that an electron exhibits a circular motion around the nucleus.
View Quiz4docx from CHM MISC at University of Florida. Immediately after filling the d sublevel of principal level n - 1 the p sublevel of principal level n is filled and the next sublevel filled will be the s sublevel of the n 1 principal energy level. A quantum mechanical system or particle that is boundthat is confined spatiallycan only take on certain discrete values of energy called energy levels.
Main Energy Levels Or Shells Sublevels Or Subshells Electron Arrangement In Atoms

How Many Sublevels Are In The Third Energy Level At Level

No comments for "The First Principal Energy Level Is Called the"
Post a Comment